Clinical studies with important results expected in 2024


| Content |
|---|
| ● Introduction (see). ● Clinical studies, important results from which are expected in 2024 (see). ● T-cell vaccine against HIV (see). ● Long-term effectiveness of malaria vaccine R21 (see). ● Additional information on this website, bibliographical references and recommended references (see). |
| In a nutshell |
|---|
| ● Nature Medicine publishes its predictions at the end of each year about which studies are most likely to yield highly significant results in the coming year. ● The selected topics for 2024 are: breast, lung and melanoma cancer, malaria, familial hypercholesterolemia, HIV infection, Parkinson’s disease, artificial intelligence in emergency medicine, children’s mental health and perinatal depression. ● Two vaccine studies are discussed: the R21 malaria vaccine and a candidate for the prevention of HIV infection. |
-ooo-
Introduction
The beginning of each year is a favorable time to formulate desires and forecasts for the year ahead. Each year, the journal Nature Medicine publishes an article summarizing eleven important ongoing studies with results expected in the new year (Arnold S., Nature Med, 2023).
A year ago, a paper in the journal Nature Medicine looked at 2023. Now, 11 expert researchers are asking which studies conducted in 2024 they think are likely to have a big impact. Among the responses, two involve vaccines: against malaria and against human immunodeficiency virus (HIV) infection. This note briefly reviews these two clinical studies from which important results are expected.
(To come back to the beginning)
Clinical studies with important results expected in 2024
The accompanying table shows the studies selected by Nature Medicine. These include research on current topics such as breast, lung and melanoma cancer, malaria, familial hypercholesterolemia, HIV infection, Parkinson’s disease, artificial intelligence in emergency medicine, children’s mental health and perinatal depression.
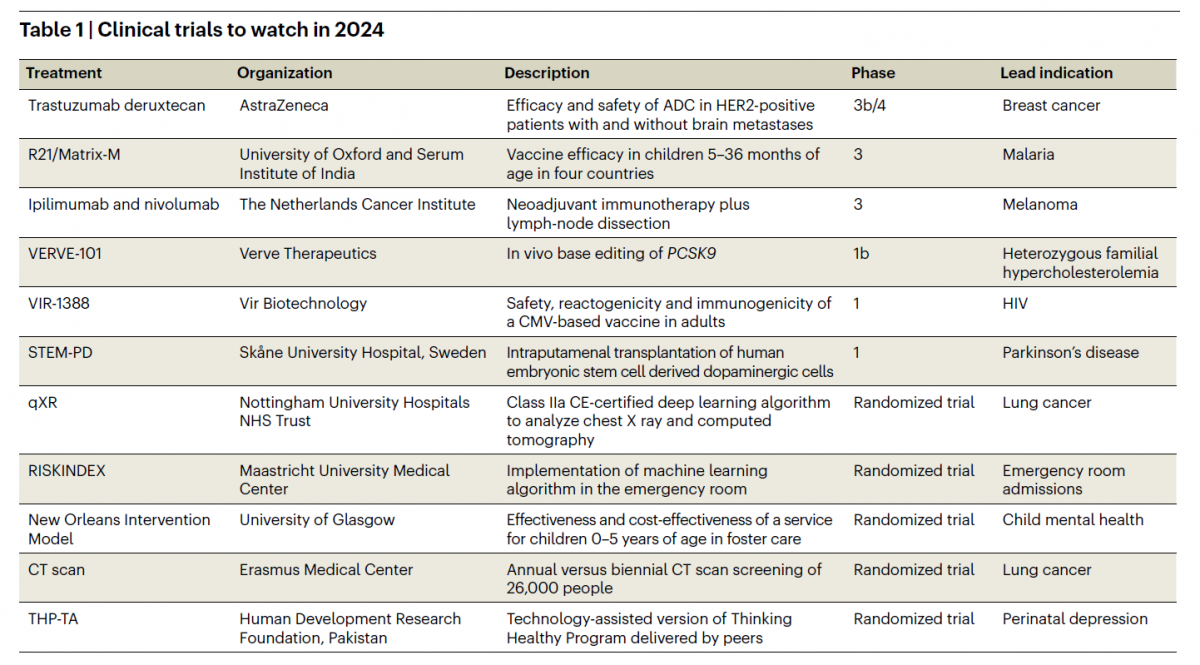
Among them, two relate to vaccines, which will be discussed below.
(To come back to the beginning)
T cell vaccine against HIV
The expert who proposed this assay is Cary Hwang (Vir Biotechnology, California, USA). It states: “The objective of our study (NCT05854381) is to evaluate the safety, reactogenicity and immunogenicity of VIR-1388, a vaccine for the prevention of HIV infection. This is a multicenter, randomized, double-blind, phase 1 study. “A placebo-controlled study enrolling healthy, HIV-uninfected adults aged 18 to 55 years who will receive one of three doses of VIR-1388 or placebo.”
VIR-1388 is a vaccine containing cytomegalovirus (CMV) as a vector that induces robust and durable T cell responses that can potentially prevent HIV infection. This follows a proof-of-concept trial of VIR-1111 that demonstrated its safety, although without a strong immune response; VIR-1388 is less attenuated than VIR-1111 and is considered more immunogenic.
The overall study design includes two parts. The first will be an initial phase involving a small number of people of childbearing age who are seropositive for the CMV vector, with careful monitoring for safety. The second part will expand participation to a broader CMV-seropositive population, including people of childbearing age (who will be required to use two forms of contraception), with safety monitoring. An additional long-term follow-up study is planned that will extend participation to 3 years after the first dose.
The corresponding doses were recently administered to the first study participants. The study is being conducted at ten sites in the US and two in South Africa and is supported by the US NIAID and the Bill & Melinda Gates Foundation. From a public health perspective, the availability of an HIV vaccine will obviously have an extraordinary impact.
Also see other news about HIV and vaccines on this site.
(To come back to the beginning)
Long-term effectiveness of R21 malaria vaccine
In this case, the answer is from Adrian Hill (Jenner Institute, University of Oxford, UK).
The main problem with malaria vaccines, and one of the reasons it took more than 100 years to develop, is that the vaccine requires extremely high levels of antibodies for it to work. Forty vaccines with the same circumsporozoite protein antigen have reached the clinic, and only two of them have shown sufficient effectiveness: RTS, S and R21. The effectiveness of the RTS,S/ASO1 vaccine drops from 55% to about 30% four years after vaccination, so long-term follow-up is really important.
A. Hill continues: “We are halfway through a phase 3 multicentre randomized controlled trial (NCT04704830) of the R21/Matrix-M vaccine against symptomatic malaria in African children. It includes standard vaccination schedules depending on age, as well as seasonal vaccination schedules used in children. from 5 to 36 months. In each group, a booster dose (fourth dose) of the same vaccine was given 12 months after the third dose. Initial follow-up will take place 2 years after the third dose, with primary analysis between 5 and 36 months of age. 12 months 2,400 participants have already registered to receive the standard vaccination regimen in endemic areas of Burkina Faso, Kenya and Tanzania. A further 2,400 participants will be registered for the seasonal vaccination scheme in Burkina Faso and Mali.”
Also: “We believe that R21 will perform better than RTS,S because R21 uses nanoparticles that have a much higher density of antigen on the surface. Vaccines such as the HPV vaccine work throughout life because of this characteristic. This research is being funded by the Serum Institute of India, which has committed to producing 100 million doses of the vaccine at a cost of US$3-4 per dose. will continue to publish more analyzes of the safety, immunogenicity and effectiveness of the vaccine.
Also see other news about malaria and vaccines on this site.
(To come back to the beginning)
-ooo-
More information on this website
Bibliographical references and recommended references
- Alpanez E. Gene editing, artificial intelligence, an HIV vaccine and more: 11 clinical trials that will define medicine in 2024. Nature magazine selects the most promising of them, which will take place next year and which could affect our health. El País, Health, December 7, 2023
- Arnold S. and others. 11 clinical trials that will shape medicine in 2024. Nat Med.2023;29:2964-8.
- ClinicalTrials.gov. To examine the safety, reactogenicity, and immunogenicity of VIR-1388 compared with placebo in participants without HIV.
- ClinicalTrials.gov. R21/Matrix-M in African children against clinical malaria.
- MS data, and others. A randomized controlled phase III trial evaluating the malaria vaccine candidate R21/Matrix-M™ in African children. SSRN. 2023, September 26 DOI: 10.2139/ssrn.4584076.
- Datto M.S., and others. Safety and efficacy of the malaria vaccine candidate R21/Matrix-M in African children: a multicenter, double-blind, randomized, phase 3 trial. Lancet. 2024;403(10426):533-44. ►Commentary: Lancet editorial. Malaria vaccines: a global health challenge. Lancet. 2024;403(10426):503. ►Comment: Nnaji K.A. and others. Vaccine R21/Matrix-M: optimization of supplies, maximum effect. Lancet. 2024;403(10426):525.
(To come back to the beginning)
