Problems with learning and memory in Down syndrome are associated with changes in the hidden part of the genome.
A team from the Center for Genome Regulation (CRG) has discovered that the Snhg11 gene is essential for the function and formation of neurons in the hippocampus. Experiments in mice and human tissue show that the gene is less active in brains with Down syndrome, which may contribute to the memory deficits seen in people with the genetic changes. The results are published today in the journal Molecular Psychiatry.
Much of the attention in genomics has focused on protein-coding genes, which in humans make up about 2% of the entire genome. But the “hidden side” of the genome includes many non-coding DNA sequences whose role in regulating gene activity is increasingly recognized, influencing genetic stability and contributing to the emergence of complex traits and diseases such as Snhg11.
This is a special type of RNA that is transcribed from DNA but does not lead to protein synthesis. This study provides the first evidence that non-coding RNA plays a critical role in the pathogenesis of Down syndrome, a genetic disorder caused by the presence of an extra copy of chromosome 21, also known as trisomy 21.
It is the most common genetic cause of mental retardation, affecting approximately five million people worldwide. People with Down syndrome have memory and learning problems related to abnormalities in the hippocampus, a part of the brain involved in learning and memory formation.
“This gene is particularly active in the dentate gyrus, one of the few areas of the brain where new neurons are created continuously throughout life. We found that abnormal expression of Snhg11 leads to decreased neurogenesis and impaired plasticity, which plays a direct role in learning and memory. and indicates that this gene plays a key role in the pathophysiology of mental retardation,” says Cesar Sierra, first author of the paper.
What is the effect of low Snhg11 levels?
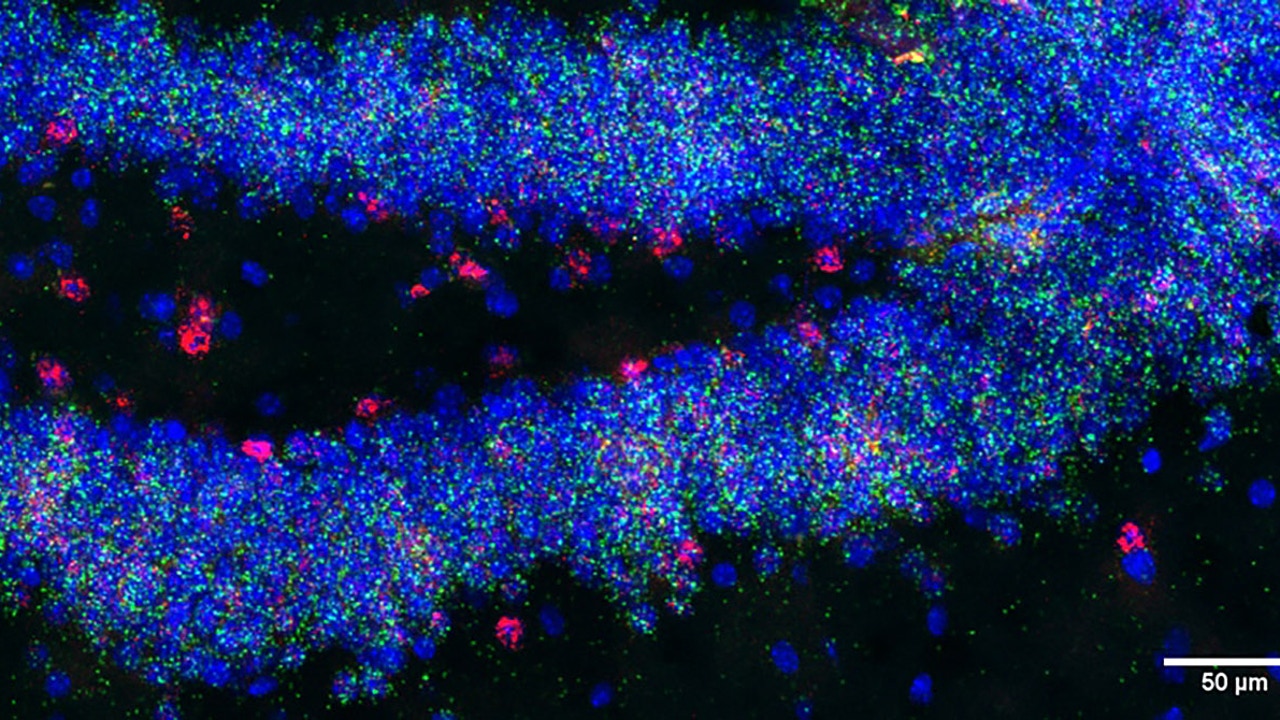
The team studied the hippocampus in mouse models that have a genetic makeup similar to Down syndrome in humans. There are many different types of cells in the hippocampus, and the study aimed to understand how the presence of an extra chromosome affects these cells.
They isolated nuclei from brain cells and used a technique called single-nucleus RNA sequencing to see which genes were active in each cell. One of the most striking findings occurred in dentate gyrus cells, where a significant reduction in Snhg11 expression was found. They also found lower levels of Snhg11 in the same types of human trisomy 21 brain tissue obtained from postmortem donors.
The experts then reduced the gene’s activity in the brains of healthy mice and found that low levels of Snhg11 were enough to reduce synaptic plasticity, or the ability of neural connections to strengthen or weaken over time. Synaptic plasticity is critical for learning and memory. It also weakened the mouse’s ability to create new neurons.
To understand the real-world implications of their findings, the researchers also conducted several behavioral tests on mice. These experiments confirmed that low levels of Snhg11 lead to memory and learning problems similar to those seen in Down syndrome, suggesting that the gene regulates brain function. Prior to this work, Snhg11 activity was only associated with cell proliferation in various types of cancer.
Pathways to new therapeutic interventions
The study authors will conduct further research to discover mechanisms of action and obtain information that could open up potential avenues for new therapeutic interventions. They will also investigate whether other genes involving long non-coding RNAs, many of which have yet to be discovered, may also contribute to mental retardation.
“There are many interventions to help people with Down syndrome live independently, but few are pharmacological. “Studies like this help lay the groundwork for finding strategies that improve memory, attention and language function or prevent the cognitive decline associated with aging,” he concludes. Mara Diersen, lead author of the study.
Reference: Sierra et al. Molecular Psychiatry, 2024.