Controversial Alzheimer’s drug banned in EU, approved in US

Last Friday, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) refused to grant permission to sell the drug Lekambi (trade name lecanemab) in Europe.intended to treat Alzheimer’s disease. It’s a controversial decision that has sparked a cascade of opinions in recent days. And it’s likely to continue to be talked about in the coming months.
In Spain, the latest to take a stand on the issue is the Spanish Confederation of Alzheimer’s and Other Dementias (CEAFA). The organisation believes that the recent clinical trials of lecanemab and other anti-amyloid drugs have marked a “turning point”. They therefore explain that they are “deeply disappointed” that People with Alzheimer’s disease in Europe ‘now excluded from access to lecanemab’“Although they are optimistic that EU regulators will reconsider their position.
“We understand that lecanemab is not a miracle cure for everyone with Alzheimer’s disease. However, the existence of the first disease-modifying drug with a new mechanism of action represents an undeniable and important advance for a field that has waited more than two decades for new drugs. Lecanemab has shown an impact on disease progression, as well as secondary endpoints such as quality of life and caregiver burden. “Rather than excluding all patients from this new treatment for safety reasons, we expected the European Medicines Agency to authorize the drug with a clear risk management plan to address potential side effects,” CEAFA said in a statement on Monday.
Currently, 6.9 million people in Europe suffer from Alzheimer’s disease.. That figure is expected to double by 2050 due to an ageing population. That’s why there’s a rush to find an effective treatment for one of the most brutal diseases in existence. The US, Japan, China, South Korea, Hong Kong and Israel have all moved to approve lecanemab in the past year. Switzerland and the UK are considering doing the same. But Europe has rejected it.
Difference in criteria between the EU and the US
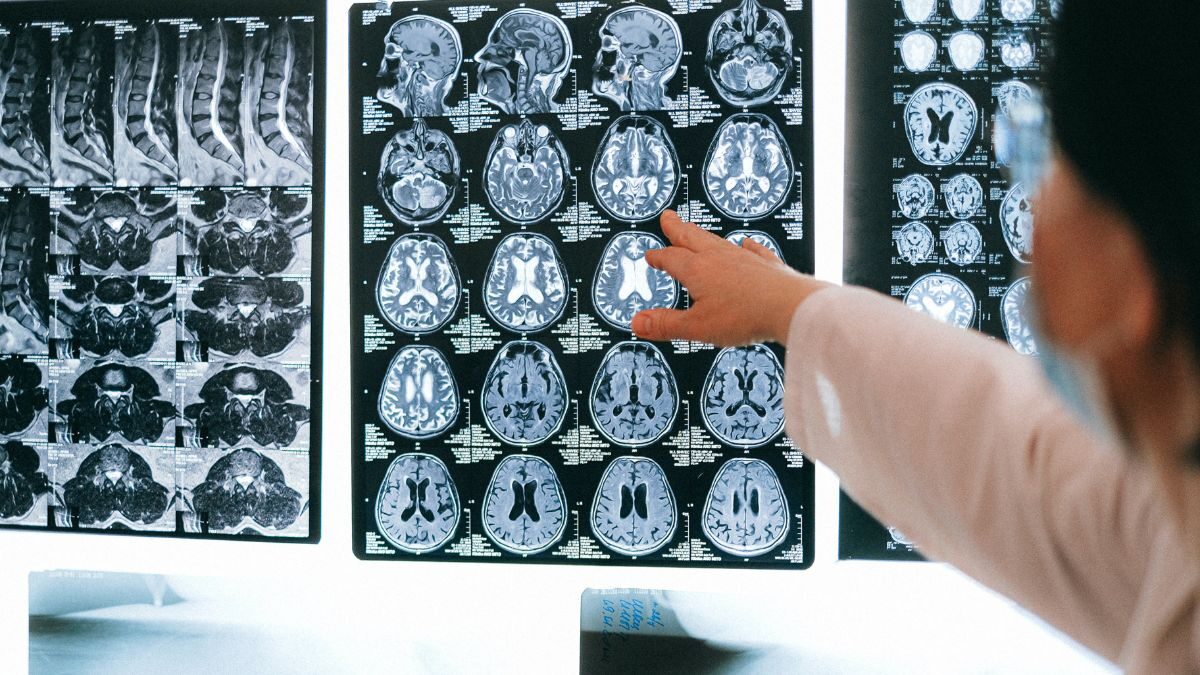
Lekembi was developed as a drug for the treatment of adults with mild cognitive impairment. (problems with memory and thinking) due to Alzheimer’s disease and early Alzheimer’s disease. It should be given as an infusion (drip) into a vein once every two weeks.
The medicine contains the active substance lecanemab, a monoclonal antibody (a type of protein) that attaches to a substance called beta-amyloid, which It forms plaques in the brains of people with Alzheimer’s disease. By binding to beta-amyloid, the drug reduces the amount of amyloid plaques in the brain and was therefore expected to delay the worsening of the disease.
According to the EMA, Biogen and Eisai (the two pharmaceutical companies behind the drug) presented results from a pivotal study involving 1,795 people with early-stage Alzheimer’s disease who had beta-amyloid plaques in their brains and were given either Lekembi or a placebo (dummy treatment). The main measure of effectiveness was change in symptoms after 18 months, measured using a dementia rating scale known as the CDR-SB.. The CDR-SB scale is used to assess the severity of Alzheimer’s disease in patients. It includes questions that help determine the extent to which cognitive impairment has affected the patient’s daily life. The scale ranges from 0 to 18, with higher scores indicating greater impairment.
The main study showed that after 18 months of treatment The CDR-SB score in patients receiving Lekembi increased by 1.21 compared to 1.66 in patients receiving placebo. Although patients receiving Lekembi had lower CDR-SB scores than those receiving placebo, the difference between the two groups was small.
“The most important safety concern with Lekembi is the frequent occurrence of amyloid-associated imaging abnormalities (AIAs).a side effect seen on brain imaging that includes swelling and possible bleeding in the brain. While most cases of ARI in the main study were mild and asymptomatic, some patients experienced serious complications, including large brain bleeds requiring hospitalisation. The severity of this side effect should be considered in the context of the small effect seen with the drug,” the European agency explained.
In addition, the CHMP was concerned that The risk of ASCVD is greater in people who have a certain form of the apolipoprotein E gene.it’s called ApoE4The risk is higher in people with 2 copies of the gene. ApoE4 who are known to be at risk of developing Alzheimer’s disease and are therefore likely to be suitable for treatment with Lekemby.
What ended up happening was that the EMA believe that the benefits of treatment are not important enough to outweigh the risks associated with it. Leckemby, so he recommended refusing marketing authorisation in the EU. They made the decision, as detailed, after consulting a scientific advisory group on neurology, which included experts and people living with the disease.
Disappointment among experts
From QMS Spain were in contact with experts from different fields to find out their opinion on this matter. And while there are nuances in their positions, the overall trend is one of concern and disappointment with Europe’s position.
“The EMA decision raises two major issues, both for the clinical, medical and healthcare community and for the researcher. Patients from Europe will be discriminated against and will not have the same opportunities as patients from other countries. And in terms of research and investment in research, Europe will also come in second place. We will go through the desert again. We will be in a complete drought again,” commented Merce Boada Rovira, neurologist and medical director of the Alzheimer Eisa Center in Barcelona.
“Lecanemab’s Phase III clinical trials showed that it does what it’s supposed to do: reduce toxic amyloid in the brain and slow cognitive decline. From a scientific perspective, this was an important step forward. However, the effect size was modest, coupled with significant side effects such as inflammation and brain bleeding, which caused death in some people.. The EMA’s decision will be disappointing to many, but there are reasons to remain hopeful. Lecanemab has shown that it can slow the progression of the disease, and research is underway. “We now need to redouble our efforts to find new, safer treatments,” said Tara Spears-Jones, president of the British Association of Neurosciences.
John Hardy, professor of neuroscience and group leader at the UK Dementia Research Institute at University College London (UCL), was more critical: “I am disappointed by the decision not to license lecanemab. The question is whether the clear statistical benefit of the treatment is worth the risk of serious, albeit rare, side effects with any treatment, and in this case the EMA in Europe and the FDA in the US have come to different conclusions when presented with similar data. We will now see wealthy people with early-stage Alzheimer’s flying to the US or other jurisdictions for treatment. “Although I suspect this decision will be reconsidered as American doctors and others gather and report real-world treatment experience.”
