“Nanorobots can improve the delivery of drugs that are currently ineffective because they have to overcome the body’s biological barriers.”

Gema Maldonado
Is it possible to send a kind of “bomb” into a tumor using nanorobots that can independently move around the organ, find the tumor, penetrate it and release drugs that destroy it? Maybe. It’s not like some sci-fi movie that came out years ago left us with visions of microships traveling through the body, but a team of researchers from Spain has achieved that its nanorobots loaded with radioactive iodine have destroyed 90% of tumors in the body . bladder in mice. Their research and results earned them publication in Natural nanotechnology.
Laboratory of Samuel Sanchez at IBEC, to which the researcher belongs Meritchel Serra, supervised the work. Both are first authors of the study, and to better understand how this technology works and what nanomedicine could mean for treating various diseases, we spoke to a researcher involved in various projects with nanorobots that could change forms of treatment and improve the effectiveness of drugs.


“Similar to the motion reaction of a car that uses gasoline to move, our nanorobots use urea to break it down and move.”
Why did you design this study specifically for bladder cancer?
Because it was an ideal scenario because the nanorobots we use are powered by an enzyme called urease, which breaks down the urea found in urine. This chemical reaction is similar to the reaction of a car that uses gasoline to move. Our nanorobots use urea to break it down and move. Thus, bladder cancer was an ideal scenario since it already contains fuel for nanorobots.
What are the characteristics of the material from which nanorobots are made, and how do you know if it is suitable or not?
The nanoparticle consists of silica, a material found in sand, for example. We use it because it’s very porous, which gives us some advantages when it comes to being able to load drugs into those pores. But we also used it because we had previous studies from 2017 in which we saw that they were able to transport chemotherapy and do so more efficiently than nanoparticles without movement, and could also penetrate small mini-tumors outside of a living being. in the laboratory. .

We saw that they worked very well and did not cause toxicity, so we continued to use this type of particle. But we are also exploring other materials, such as those already approved by the European Medicines Agency or the FDA in the US, to further facilitate the transition to clinical trials.
“Our technology consists of three parts: the “chassis”, which will be the nanoparticle, the “motor”, which will be the urease, and the therapeutic agent. These three parts are easy to mold and adapt to any situation.”
So, will they be able to change the type of material in the next stages of the study?
Precisely because we have a technology consisting, so to speak, of different “packages”. One is the “chassis”, as if a car had one, and in this case it would be a silica nanoparticle, but it could be made of a different material, we could change it. Then there is the part that acts as the “car engine”, which is the urease, which will continue to provide the same motor effect. And thirdly, we have a therapeutic agent that we can change as well.
In this case we used radioactive iodine, but previously we have also used chemotherapy, which we are simultaneously studying in trials in mice. Thus, these three technologies are quite amenable to shaping and adapting to any situation.
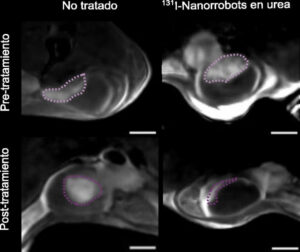
What causes nanorobots to target a tumor and release the radiation therapy they carry inside?
We have seen that when urea, which is present in urine, decomposes, a change in pH occurs, and this change, which is very localized because it occurs around the nanorobots, is capable of destroying the extracellular matrix, that is, the tumor wall, when the nanorobots approach it. This is why they penetrate the tumor and not healthy tissue. This is the mechanism due to which up to 95% of nanorobots accumulate in the tumor and only 5% in healthy epithelium. Thus, the radiotracer they carry only acts in the area of the tumor.
“In this case we used radioactive iodine, but previously we have also used chemotherapy, which we are studying in parallel in trials in mice.”
With one dose, 90% of the volume of these tumors was reduced in the study mice. Are you wondering if the second dose will kill the tumor?
At the same time, we are conducting other studies to see how many injections are needed to completely destroy the tumor, and to check whether there are relapses, that is, whether the tumor appears again after some time.
What are the next steps in this investigation?
In January 2023, our research team created Nanobots Therapeutics, a subsidiary of the group, to try to bring this technology to market. And the first step is the opportunity to participate in clinical trials. To do this, they are raising capital and asking for funding so that they can get through all the steps in the regulatory process and all the preclinical studies that are missing once we have the evidence we need to conduct clinical trials.
“We estimate that in about four to five years we will be able to conduct clinical trials of nanorobots.”
We estimate that in about four to five years we will be able to conduct trials because the radiopharmaceutical has already been tested in thyroid cancer; If we replace the “chassis” with a material that is already approved by the regulator, and demonstrate that the combination of these elements is safe, it will certainly make our job of achieving phase one testing easier.
Can they help with other types of cancer?
If only it were possible. As part of my doctoral dissertation, I am involved in a project with the Vall d’Hebron Hospital in Barcelona, where we are trying to use nanobots, although in a slightly different way, because we change the “engine”: since we do not have urea in the rest of the body, we we use another enzyme that also allows us to propel nanorobots. We always perform these procedures locally, meaning they are not injected into the bloodstream intravenously or travel through the bloodstream. Now we are studying them for tumors of the gastrointestinal tract.
“We are researching materials that are non-toxic and good drug carriers, since many of them are very difficult to dissolve in water.”
What are the implications for nanomedicine of finding safe materials that can be good vehicles for delivering drugs to the area where they are needed?
We are most interested in two factors: on the one hand, they are non-toxic, do not cause side effects, and on the other hand, they are good carriers, since many drugs are very difficult to dissolve in water. For example, in the Moderna vaccine against Covid-19, they used lipid nanoparticles carrying RNA inside them. In each case, we can change the material of the nanoparticle to tailor it to the drug it carries. Thus, if we have a drug that is poorly soluble in water, we need a nanoparticle that can encapsulate it and is highly soluble.
How do you think nanomedicine will impact approaches and treatments for diseases in the coming years?
In all those situations where current drugs are not effective enough because they have to overcome biological barriers in the body, such as the mucus barrier or the blood-brain barrier, nanomedicine can improve drug delivery. At the same time, since the drug is not exposed, it can significantly reduce its side effects.
“Because drugs in nanorobots are not exposed, their side effects can be significantly reduced.”
Will this allow us to move towards more targeted and more personalized therapy?
This is true, but large investments are needed in the development of nanomedicine that is most suitable for each type of disease, because each person has his own complications and his own treatment. We won’t have a single nanoparticle that cures everything, or a single nanorobot that can cure every cancer, but we must continue to research. But they are very promising.
