They are observing at the atomic level the neuronal gateways for molecules needed for learning and memory.

 Maria Martínez Molledo, first author of the paper, and Oscar Lorca, co-author, researcher and group leader of macromolecular complexes in DNA damage response at CNIO. Photo: Esther Sanchez/CNIO.
Maria Martínez Molledo, first author of the paper, and Oscar Lorca, co-author, researcher and group leader of macromolecular complexes in DNA damage response at CNIO. Photo: Esther Sanchez/CNIO.
The Asc-1 protein is a neuronal entry (or exit) pathway for essential amino acids in cognitive processes. New work now reveals its structure and mechanism of action.
It is a collaboration between CNIO, IRB Barcelona, University of Barcelona and CIBERER and is published in the journal Nature Communications.
The discovery could be used to develop drugs against schizophrenia, stroke and other neurological diseases.
Learning an experience, remembering a joke, changing an attitude… all our behavior is the result of the exchange of chemical compounds between neurons – neurotransmitters. Finding out exactly what happens at the molecular level when neurons they say among themselves, at synapses, is essential for understanding the functioning of the human brain in general and, in particular, for helping to solve mental health problems.
A new study was able to observe and describe the structure of a protein present in the membrane of neurons, a protein that acts as a gate that opens and closes. It acts as a specific transporter of certain key amino acids necessary for learning and memory. This is the Asc1/CD98hc protein, abbreviated as Asc1.
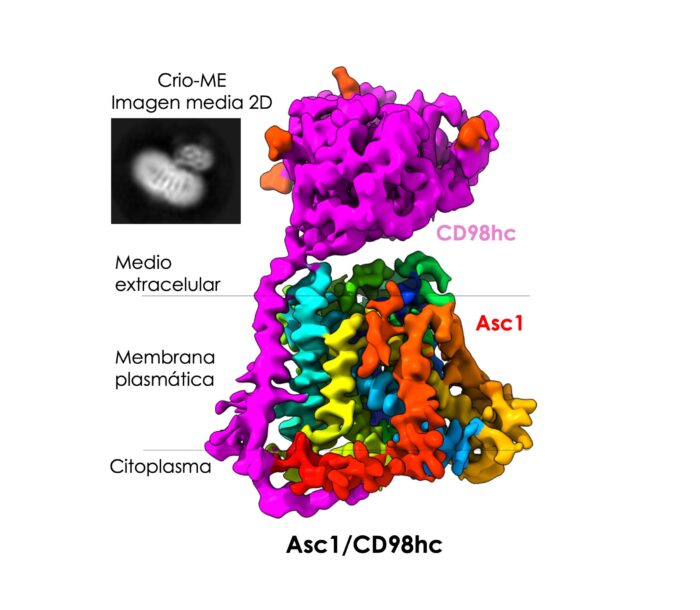
Inset: Electron microscopic image showing the CD98hc component protruding from the membrane and the transverse bands corresponding to the Asc1 protein.
The activity of the Asc-1 protein is associated with various types of mental illnesses, and knowledge of its three-dimensional shape will allow the development of new drugs for these pathologies.
explains this Oscar Lorca, from CNIO: “Modulation of Asc-1 activity may be a therapeutic strategy for conditions such as stroke and schizophrenia. “Determining the structure of Asc-1 at atomic resolution is important because it can help in the search for compounds that alter its activity.”
“The collaboration between IRB Barcelona, CNIO and UB has been key to unlocking the mysteries of Asc-1, offering us unprecedented insight into its structure and function. “This discovery not only sheds light on the complex cellular machinery underlying fundamental cognitive processes, but also brings us closer to developing more precise therapeutic interventions for a range of neurological disorders,” he adds. Manuel PalacinHead of the Laboratory of Amino Acid Transporters and Diseases at the Barcelona IRB and Professor in the Department of Biochemistry and Molecular Biomedicine, Faculty of Biology, UB.
In addition to Oscar Lorca and Manuel Palacin, he is a co-author of this work. Ekaitz Errasti-Murugarren, from the University of Barcelona and CIBERER. First signatories Josep Rullo-Tubau (IRB Barcelona) and Maria Martinez Molledo (CNIO).
Funding comes mainly from the La Caixa Foundation and the Ministry of Science, Innovation and Universities. Effect on neurological diseases.

All cells of the body have in their membrane gateways for the exchange of substances with the external environment: proteins that constantly open and close in accordance with the needs of the cell. They open inward, take, for example, an amino acid and, changing its shape, release it, opening outward, or vice versa.
The Asc-1 protein is found predominantly in neurons of the hippocampus and cerebral cortex, in the brain. It specializes in introducing and/or removing from a neuron two essential amino acids for neural connections (synapses) involved in learning, memory and brain plasticity – the ability of the nervous system to change its circuits in response to a new environment.
Fluctuations in the supply of these amino acids, called D-serine and glycine, are associated with schizophrenia, stroke, ALS and other neurological diseases. Efforts have been made for some time to develop drugs that modulate Asc-1 activity to treat these diseases, but have been unsuccessful. Detailed knowledge of the atomic structure of Asc-1 provides key information for achieving this goal.
Hunted when it opened inside
The Asc-1 protein was purified by Josep Rullo-Tubau at the Barcelona IRB and transferred to the CNIO so that Maria Martinez-Molledo could observe it using cryo-electron microscopy and with these images she could determine the structure of Asc-1. in 3D and high resolution. In cryo-electron microscopy, molecules are frozen at high speed and observed in electron microscopes; Advanced visualization techniques are then used to interpret the information.
The observed structure shows Asc-1 when it is trapped at a stage in which gates It was open to the inside of the cell, waiting to receive an amino acid for transport.
“From its atomic structure, we were able to predict which parts of the protein seemed important for binding the transported amino acid, as well as the possible mechanism for transporting it outside the cell,” says Llorca.
Groups Victor Guallar (Barcelona Supercomputing Center) and Lucia Diaz (Nostrum Biodiscovery) made these predictions about transporter function, which were tested by Rullo-Tubau, by measuring the effect of specific mutations in Asc-1, which were complemented Rafael Artuch (Hospital of Sant Joan de Déu) and the scientific platform of biostatistics and bioinformatics of the IRB of Barcelona, which leads Camilla Stefan-Otto Attolini.
Two course of action
The findings also help explain another feature of Asc-1. The rest of the family of transporters it belongs to, called HATs, can only transport one amino acid into the cell when they take another, and vice versa. That is, they function only through the exchange of amino acids. Asc-1, however, can also extract an amino acid without the need to introduce another and open and close in a vacuum. This mode of activity is called diffusion.
The results obtained from the molecular structure of Asc-1 provide insights into the function of each transport mode.
Return to news